 |
| Source - https://xkcd.com/2658/ Rollover text: Theoretical physicist: At the Planck length, uncountably many. |
See, it's funny because...
I'll leave the middle three panels (and the rollover text) to the fine crowdsourcing folks at explainxkcd. If you don't get those, check the link.
...but the chemistry one is all mine.
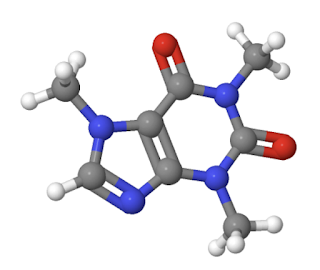
The caffeine molecule...
...is C8H10O2N4 (the grey spheres are C, white are H, red are O, and blue and N).
There are two rings in the structure, so that's two holes per caffeine molecule.
If there are "1021 [holes] in the caffeine alone", that would mean 5 x 1020 molecules of caffeine or 0.16 g caffeine.
From healthline.com, "You can expect to get around 95 mg of caffeine from an average cup of coffee. However, this amount varies between different coffee drinks, and can range from almost zero to over 500 mg."
That calculation of 0.16 g = 106 mg then seems about right.


No comments:
Post a Comment