This video surveys the work being done to create superheavy elements - from the Glenn Seaborg era through today. It's a good start toward understanding how new elements are created, though I would also recommend the book Superheavy - a copy of which I have on my shelves at school.
December 29, 2025
December 22, 2025
How to make Coca Cola
This sounds like a much more expensive, labor-intensive method of producing homemade Coca Cola.
...but it would give you big hipster, crunchy nuts and berries vibes, though, and it would be really cool to do if you have the time, money, and soda water.
December 15, 2025
How the Demon Core Killed a Man in 9 Days
The demon core is a legendary sphere of plutonium gallium alloy that was designed to be - when encased in neutron reflectors like beryllium or tungsten - just a hair away from achieving critical mass and releasing massive amounts of radiation.
That idea of making something that is just a tiny accident away from releasing deadly anything was - in retrospect - perhaps a mistake.
The demon core became known as such because of two deadly incidents, the second of which is retold in the above video, fictionalized in the below clip from the movie Fat Man and Little Boy, and more thoroughly examined in the third video from Kyle Hill.
Check all the videos out and maybe remember not to build a gun that shoots bullets out in every direction while giving it a hair trigger, eh?
December 8, 2025
The Unknow Phase of Matter
God lord but Steve Mould is a brilliant communicator of science.
I've never really understood super critical fluids - even though I've posted about them before, but today's video - especially the part from about 10:20 through 14:20 or so - makes for a brilliant explanation of why fluids go supercritical at higher temperatures.
The use of the entropy and potential energy graphs in combination lays out the argument for supercriticality brilliantly.
Thanks, Steve...thteve.
December 1, 2025
AtomAnimation
That right there is a screenshot from AtomAnimation, a website that seems to do nothing more than provide animations of Bohr models for any element with any combination of protons, neutrons, and electrons.
It's kind of fun to play around with, though I warn you that the larger atoms take a decent amount of time to render and seemed to slow my browser down a bit while they were running.
November 28, 2025
Stranger Things S1 Chemical Mystery
If you know me, you might know that I'm the kind of guy who would notice a scientific issue while watching a movie or tv show.
I didn't, however, catch the anachronism of a 2000s periodic table in season one of the 1980s-set Stranger Things.
Clearly, ten or so elements (#108 and #110-118) - those discovered after 1983's setting for the first season. Another couple of them would need to be presented in three-letter, temporary names because the controversies around the names for elements #105-109.
In all, I don't think the periodic table anachronism caused audiences to enjoy their Stranger Things any less.
November 10, 2025
This bizarre density toy just got an upgrade
I have the 'original' polydensity kit from Ed Inn (now Flinn, blech), and it's a fascinating toy.
Two insoluble liquids and two types of beads - pony and UV - which make for some fascinating behavior when the bottle is shaken and allowed to settle and separate.
In today's video, Steve Mould attempts to make a similar bottle with three insoluble liquids and four polymer beads.
He does achieve his desired result by the end, but the beads he uses look to be a little tougher to source.
Sadly so...
September 29, 2025
Why Lithium Is Dangerous But PERFECT For Batteries
Our chemistry book has a diagram of a battery in the electrochemistry chapter, and I discuss that battery for a bit before explaining to my students that the basics of ACME (anode, cathode, metallic path, electrolyte) hold for every battery but that the engineering of modern lithium-ion batteries is far different from the diagram in the book.
This video - again leaning into the algorithm-rewarded longer and longer format - explains some battery basics involving the activity series, the history of the development of the lithium-ion battery, and the methods of fiery failure when the battery overheats.
This is, as Dr Derek says, a technology that has allowed our modern, battery-dependent world.
September 22, 2025
H and Cl sitting in a tree
I pretty consistently tell my students not to personify atoms and molecules. They don't have hopes and dreams. They don't fall in love. They simply move to more stable arrangements.
...but...
It's very cute to think of H and Cl being in love and holding hands, so I'm going to let Google slide on the cuteness factor alone.
September 15, 2025
Units matter
 |
| Source - Reddit |
It's similar to one that Steve Mould brought up in a video I previously posted on the blog.
To say that the temperature is tripled is incredibly vague. As I teach in the unit of gas laws, to discuss temperature tripling (or doubling or whatever) we have to consider things in their absolute temperature.
Doubling a temperature in Celsius or Fahrenheit would just double the portion above an arbitrary zero (freezing water for Celsius or the freezing point of a water/ice/ammonium chloride solution for Fahrenheit). That wouldn't really double the thermal energy or the average kinetic energy of the object because there's a whole bunch of that below the arbitrary zero.
To actually double the thermal energy or average kinetic energy, you would have to double the absolute temperature, something measured in either Kelvin or Rankine scales.
So it looks like Duolingo doesn't understand that aspect of temperature.
September 8, 2025
Where is he? We'll never know.
 |
| Source - Brewster Rockit |
See, it's funny because it's a reference to the Heisenberg Uncertainty Principle in which - in a very simplified format - we can know the momentum or position of a particle but never both.
I warn you, the uncertainty principle is a whole lot more complicated in its real form, but unless you're really into quantum mechanics, I recommend that you take the simple version instead.
With larger objects - say a person or a baseball - we can know the two quantities about the object almost perfectly, but as the object gets smaller - like atoms and protons and electrons or a shrunken Brewster Rockit - we can know less and less about the combination of the two quantities.
I get it. It takes some subtlety and understanding of the world of quantum mechanics to get the joke.
Luckily, I get the joke...like I got this other, similar one.
September 1, 2025
That's hydride.
 |
| Source - SMBC |
See, it's funny because...well, honestly I don't necessarily understand why it's funny in a scientific sense.
I get that - as is often the case in many genie stories - the genie fulfills the wish as spoken but not necessarily as the person intended it to be fulfilled. It's a fairly common trope, donchaknow.
But there are hydrogen atoms with two electrons or at least hydrogen ions that have two electrons. I guess changing every hydrogen atom to hydride ions (hydrogen with two electrons) would cause some problems, but I don't necessarily know that all of that would lead to immediate explosions.
August 25, 2025
Gimme two more!
See, it's chucklesome because the general rule is that atoms need eight valence electrons to be stable.
An atom with six valence electrons would need two more valence electrons to be stable.
So they'd act like a guy yelling at the Subway worker for two more cookies.
If you want to know the NSFW context of the video, check it out here.
August 18, 2025
Glass bottle explosion injures students, chemistry teacher at Southport High School
The really terrifying aspect of this story to me is that I've taught in that science lab.
It's a great lab space, huge and light and airy. It's a single lab space that all of the Southport High School science teachers share and that I've used to teach our summer ASM camps. I'm hopeful that Mark Duncan - who was a great host to us for our camp - isn't the teacher mentioned in the various stories. He was more of a physics than a chemistry teacher, but none of the articles mention the teacher by name, so I can't say for certain whether Mark was or wasn't involved.
 |
| Source - msn |
The woosh bottle is a demonstration that I've done countless times throughout my career. It involves putting an amount of alcohol - typically about twenty milliliters of ethyl alcohol, though other alcohols can be used - into a large bottle. The bottle is then sealed with a hand and rolled around to allow the alcohol to evaporate. The bottle is temporarily capped - in my case with a 250 mL beaker - and a source of ignition is introduced into the bottle when the cap is removed. I've used lighters or matches held by tongs or by my fingers. Early in my career, I even let a student drop the match into the bottle.
 |
| Source - The Journal Rewired |
I use plastic bottles and now put the bottle behind a safety shield because of an incident I had once where the plastic bottle fell off of the lab table onto the floor in front of students sitting too nearby for my comfort. The plastic bottle is hopefully more safe than the glass bottle from the Southport story, and the safety shield makes sure that any consequences of an explosion would stay toward the demonstrator (me) and not toward the students.
...but every demonstration deserves to be reevaluated from time to time to decide if it's a demonstration worth doing. If the safety risks outweigh the educational benefits or not.
August 11, 2025
Shadows from the Walls of Death
 |
| Source - 99% invisible |
August 4, 2025
The original liquid smoke controversy (it's fine, btw)
Big fan of Adam Ragusea here.
He does a great job balancing useful cooking information, science, and his perspective on the sometimes overreaction to and misunderstanding of that science. He's also really good at integrating the sponsor ads smoothly.
In this video, Adam goes through what's in liquid smoke, how you can make it at home, and how you can use it in food.
I'll warn you that this is a much more complex look at the side products of incomplete combustion, something we barely touch upon in our high school chemistry courses at Princeton. I do mention that it's officially complete combustion that produces just carbon dioxide and water vapor but that incomplete combustion can produce other products like carbon and carbon monoxide.
July 28, 2025
Making ULTRA-BRIGHT GLOWING GOO
Barnaby Dixon is an impressive puppeteer that I first heard about probably ten years ago. If you haven't checked out some of his work, do yourself a favor and spend a few minutes there first.
In this instructional video, Barnaby explains how he uses a thermoplastic polymer - a polymer that becomes flexible when it's heated above some temperature but is rigid below that same temperature. It's a great example of the polymer's glass transition phase change. Because polymers are mixtures, they don't necessarily have definite melting temperatures as pure substances like elements or compounds do. Instead they have ranges of temperatures during which they aren't quite solid or liquid but are flexible and moldable - think of hot glass being shapeable but not liquid.
I have some of this at school. It used to be available from Educational Innovations. That's where I bought it, but they sadly don't carry it anymore. Of course, just about anything is available at Amazon if you search for pcl moldeable plastic.
July 21, 2025
How to Make a Matchbox Rocket Launching Kit
I made paperclip-rubber band bows and arrows when I was in high school. They would launch paperclips maybe ten feet and with absolutely no consistent aim at all.
This is a few quantum leaps better than my amateur armory.
Be safe out there and launch these outside and not at people...but do make them.
July 14, 2025
World's Roundest Object!
Be careful when discussing this video with your students. This is about the world's roundest sphere...sphere. It's not a ball. It's a sphere.
Just saying.
'Cause I know I've said ball in front of my high school students, and it took me a while to get the boys' attention back.
(In a related aside, my wife is listening in as this video plays. She lost control at 6:22 when the narrator said "Newtons, Joules". To quote her: "heh, heh...Newton's jewels...heh, heh".)
This 'world's roundest object' is more about the basis of our metric (now the systemé international d'unités or SI) than it is about materials science. There are, however, some serious challenges involved in making a material that won't decompose, that won't get dirty, that won't wear away, that won't change over time.
Here they have created - according to the scientist at 7:25 - a single crystal of silicon with 'no voids or dislocations' and containing only one isotope of silicon, making the material just slightly less valuable than absolutely, perfectly, priceless.
July 7, 2025
Complete History of the Avogadro Number
Around 0:50 into the video, our meandering narrator mentions that he wasn't able to find a single source with correct, complete information on the history of the Avogadro Number...which makes me wish he'd provided sources for his information. Otherwise, how do we know that what he's telling us is correct?
(As an aside, I've always called it Avogadro's Number. Maybe the the and lack of possessive is a British thing. I'll admit that I don't remember how my professors at the University of Aberdeen referred to it.)
I will say that this is an amazingly thorough and likely correct history of the development of the concept and number of the moles. It goes through from Democritus through the Karlsruhe Congress and all the way to the modern measurement of Avogadro's number via a nearly perfect sphere of silicon.
I need to watch this video a few more times until I'm more familiar with all the steps in this development so I can tell the story to my students.
June 30, 2025
Making an atomic trampoline
I've said it before, but an atomic trampoline demonstration set-up would make for a spectacular gift for your favorite neighborhood blogger.
NileRed took a different route than I've taken - which is mostly just wishing that I would stumble across an atomic trampoline and not really doing anything at all to make that happen - and decided to make a disk of amorphous metal on his own.
Admittedly, one of our ASM Master Teachers has a lead on getting sets of amorphous metal disks for us to have in our classrooms. It involves the material scientists at Apple's headquarters in California and turned out to be much more complicated than expected because - as NileRed finds out - the adhesive used to affix the amorphous metal to the steel base is highly relevant in maintaining the ridiculously bouncy nature of amorphous metals in this application.
Here's to hoping that my strategy of doing nothing and just hoping things will work out will...um...work out.
I'll include the Grand Illusions videos that inspired Steve Mould's video that in turn inspired NileRed's above video...
June 23, 2025
Why Are Soap Bubbles So Colorful?
The wave-particle duality of light is mind boggling to me, though it's not that impressive to most of my students.
The pretty colors of a soap bubble, on the other hand, unite us both in going, 'ooh, pretty."
June 16, 2025
How One Company Secretly Poisoned the Planet
At some point in my material science and chemistry courses, I speak bluntly to my students that most research suggests that man-made polymers are bad for us.
Some are worse than others, but most research on the effects of polymers on humans seems to suggest that there are bad effects from most man-made polymers. Some are minorly bad, but others - like the family of PFAS - are more obviously and persistently bad.
The video above is short and has a direct message: DuPont is bad (or has acted badly).
The longer video below - from Veritasium - is far longer but is much, much more informative.
If this sounds familiar, you might've seen a semi-recent movie about this story, Dark Waters.
June 9, 2025
5 Regrettable Things People Did With Uranium
Here's the list...
- Uranium glass (not actually on the list but mentioned in the introdution)
- Dentures - The uranium added to the porcelain in the 1940s helped the 'teeth' look more natural - including absorbing ultraviolet radiation like natural teeth do.
- Toys - I recognize that chemistry sets from the mid-20th century weren't remotely safe, but the current 'chemistry sets' might have swung a bit too far the other way.
- War - Not just atomic bombs here but also depleted uranium used in tank shielding and armor-piercing rounds. I've posted about this use before.
- Medicine - I hadn't heard about this use of uranium to treat diabetes before, but it sounds like it wasn't useful and was even harmful. Might want to check that whole Hippocratic thing.
- Spas - Tom Scott had a video on these spas - which sound like really bad ideas to me.
June 2, 2025
This 200-Year-Old Lighter Ignites Without a Spark
I understand that the act of creating fire at this point in our lives is trivial.
I have a couple of piezoelectric grill lighters and a flint-based lighter in the a drawer in my kitchen. The 'pilot lights' in my gas stove, hot water heater, and furnace are all piezoelectric. If I need fire, I can have it in about two seconds.
That hasn't always been the case, of course. From prehistoric times when fire meant warmth and digestible food, the act of fire creation was miraculous and to be protected.
So the development of a 'safe' lighter that could create fire at the whims of the lighter's owner was an important step toward taming that fire.
But I certainly wouldn't want to carry around Steve Mould's Döbereiner's lamp (yes, the same Döbreiner as the periodic triads) in my pocket.
May 26, 2025
XKCD 3094 - Mass Spec
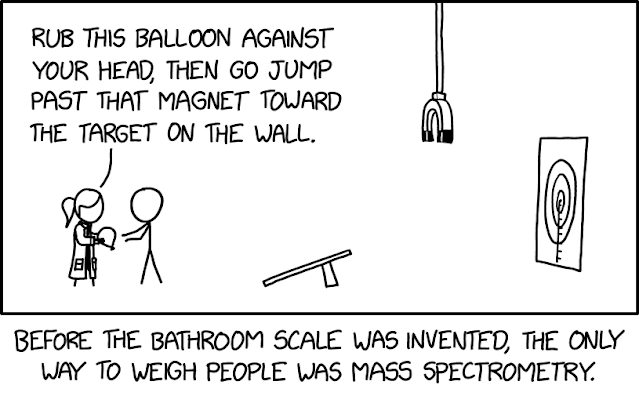 |
| Source - XKCD |
See, it's funny because this is a very rudimentary version of a mass spectrometer.
The person is the aerosolized sample atom which is ionized via balloon charging, accelerated by running and jumping, deflected via the magnet, and detected by position on the target on the wall.
It's almost exactly how mass spectrometry works...only ridiculously so.
May 19, 2025
Student Submitted - Hund's Rail
 |
| Source - C&EN |
Today's post was submitted by Katelynn J, one of my AP chemistry students. As a sub assignment after the AP chemistry exam, I had students submit two posts for this blog. I'll be posting them over the next few weeks.
During COVID-19, this cartoon was probably especially relatable, since everyone was trying to stay as far away from each other as possible. Of course, staying healthy and safe is much more important than adhering to the buddy system, but when going into two separate cars, you’re sure to miss your travel companion at least a little.
In this specific comic, however, the problem isn’t social distancing, it’s Hund’s Rule. On the rail, representing the shells of the QMM, electrons after first fill up all empty spaces before being able to double up, since this stabilizes the atom. The two electrons here trying to enter a car together would make the atom unstable, so it’s better they stay apart.
Katelynn added that "I learned that Hund has more than one rule! His rules are best for the ground state of an atom, and all three have to do with electrons," and she found this website helpful for further reading.
May 16, 2025
Student Submitted - The chemistry of papal smoke
Today's post was submitted by Katelynn J, one of my AP chemistry students. As a sub assignment after the AP chemistry exam, I had students submit two posts for this blog. I'll be posting them over the next few weeks.
Following the death of Pope Francis, a new pope now needs to be chosen. 16-20 days after the mourning period for Francis, elections have now begun and ended, and cardinal Robert Prevost, now Pope Leo XIV, has been chosen. During this selection period, all cardinals under the age of 80 travel to the Vatican to vote, and they are isolated from society until a decision is made. In order to keep the outside world updated, after every vote, ballots are burned and either black {indicating no decision has been made} or white {indicating a pope has been elected} smoke “billows” from the chimney of the Sistine Chapel. Interestingly, the longest conclave took 3 years, following the death of Pope Clement IV in 1268.
But fire only produces one color of smoke, normally. So how are the two colors created? Originally, white smoke was made by the burning of the ballots and the addition of dry grass for a lighter smoke color, and black smoke was made by ballots, wet straw, and rosin to darken the smoke. In modern times (after 2005), a mix of chemicals are used for each color. Chemicals listed here.
This chemical cocktail works because of the state of matter changes that occur. When a substance, like the chemicals used, is vaporized (fire does that!), the particles will recondense in the air, blocking light and only allowing some colors through, the color of smoke that we see.
Learn more about how other non-white/black colors of smoke are made at this link.
May 14, 2025
Student Submitted - Fruit browning
Today's post was submitted by Aleeyah B, one of my AP chemistry students. As a sub assignment after the AP chemistry exam, I had students submit two posts for this blog. I'll be posting them over the next few weeks.You know when you cut a piece of fruit and it turns brown after a while, or a banana peel? This article explains how to stop this from happening. This is actually because of a chemical reaction called oxidation. Fruits contain an enzyme called polyphenol oxidase, also called PPO. When the fruit is completely intact, everything stays inside the peel. However, if the fruit is bruised or cut, oxygen in the air goes into the fruit. The enzyme I mentioned before, PPO, reacts with oxygen and turns into something called melanins. This causes the browning we see. This process is called enzymatic oxidation. So the article just explains ways to slow this oxidation reaction.
I found it interesting that fruits turning brown does not necessarily mean that it is going bad. I always viewed brown fruits as spoiled, but now I might view that differently.
Learn more about fruit browning at this link.
May 12, 2025
Student submitted - Saponification
Today's post was submitted by Aleeyah B, one of my AP chemistry students. As a sub assignment after the AP chemistry exam, I had students submit two posts for this blog. I'll be posting them over the next few weeks.
We use soap every day, I hope, but we rarely stop to think about the chemistry of it. This source tells us that soap is made when a fat or oil made of triglycerides (fat found in blood) reacts with a strong base such as sodium hydroxide. This reaction breaks down the triglyceride molecules and makes something called glycerol (a sugar alcohol) and what we call soap. Every soap molecule has two ends, one hydrophilic and one hydrophobic. The hydrophobic end attracts to the oil and grease (non-polar), while the hydrophilic side attracts to the water (polar). As we wash our hands, the soap is able to grab the oil, but is also able to be washed away by the water.
Something that I thought was funny was that soap doesn’t just murder all the germs on your hands, but actually washes them away. I always thought soap instantly killed any bacteria it touched, and not necessarily washed them away.
You can learn more about saponification at this link.
May 5, 2025
Glittering war zone halos named for fallen heroes
Stick with me, folks.
I wouldn't normally turn to Fox News for my science reporting, but their story does the best job - of the ones I quickly searched on YouTube - of explaining the Kopp-Etchells effect in which the edges of helicopter rotor blades get abraded via airborn grains of sand, sending showers of pyrophoric tittanium-nickel alloys into the air and making for a lovely light show - that admittedly shortens the lifespan of the blades and advertises the presence of the helicopters at night.
Effectively, it turns the edges of the blades into sparklers.
It makes for some really pretty pictures, though...
 |
| Source - reddit |
 |
| Source - wikipedia |
Here's another video with a little more science presented in meme-ified format.
April 29, 2025
Put the periodic table in order - game
 |
| Source - YouTube video that has nothing to do with the linked game |
Sorry about yesterday's downer.
Try something fun and see if you can put the periodic table elements in order.
In this game - linked here - you're presented four element names, and you're supposed to click on the element that comes next on the periodic table.
For example, you'll start with four options and should choose Hydrogen because that's the first element on the table. From there, you just choose which of the four presented elements comes next.
I've only played it once, and I got knocked out at #62 - which made me kind of sad, I'll have to admit.
(And please don't buy the linked NFTs of the elements. NFTs are dumb.)
April 28, 2025
The Bhopal Gas Tragedy
I actually remember the story of the Bhopal accident appearing on the news when I was a child. I would've been about 9 1/2 years old at the time.
There's nothing but tragedy in today's story, an industrial accident in Bhopal, India where a Union Carbide plant producing two pesticides had a huge leak leading to more likely more than ten thousand immediate deaths.
There's not a huge amount of direct chemistry in today's video - in spite of a couple of chemical structures being shown - but there is something I want to say.
(Warning...heavy soapbox moment here...feel free to skip this if you don't want to hear Mr Dusch's very heavy handed opinion.)
We can never have enough corporate oversight by government inspectors.
There are people who will tell you that corporations can police themselves, that they will always take care of safety because it's their people who are in danger.
That's an absolute lie.
Corporations are concerned about profits over everything else. That's it. That's all.
Every corporation lobbying to cut regulations on their production control, every corporation arguing that safety constraints are hurting their business, every corporation trying to cut environmental laws because it hurts their business is putting your life at risk.
They don't care about your life or mine.
They care about profit.
(Oh, and happy fiftieth birthday to me today. I probably should have chosen something a little more happy and light-hearted for today's topic.)
April 21, 2025
Lake Nyos disaster
Now that's a mysterious introduction.
I assume that the full NatGeo documentary goes on to explain what happened near Lake Nyos that day, but the clip I embedded above - the only one I found from NatGeo on YouTube - cuts off before an explanation.
I'll let you make a guess as to what caused all the deaths before you click through to more detailed videos after the jump.
April 15, 2025
Mpemba effect
Yesterday I mentioned the real phenomenon of ice droplets freezing from near boiling before hitting the ground if thrown up into really cold air.
Today, some videos of that happening along with the scientific explanation of the Mpemba Effect.
The above video is one of the prettiest I've ever seen of the effect, though there are others - after the jump - that explain the science better.
April 14, 2025
Ice Spiral Math
April 7, 2025
How Does the Space Cup Work?
We've seen how water in space behaves oddly - and sometimes problematically.
The space cup works with surface tension to keep the liquid in the cup and allow astronauts to drink from an open container rather than from a straw attached to a baggie of liquid.
If you want one, you can buy one for only about $750 - but you could probably print one more cheaply than that.
March 31, 2025
Americans Have No Idea How Much Fuel Idling Uses
There's a relatively new development in cars that I've never quite understood: start-stop systems.
That's when stopped cars - at a red light, for example - automatically turn their engine off and start up again when the driver tries to get the car moving again. According to this video - which cites research articles - it only takes about seven seconds of idling to use up more fuel than it takes to simply turn the car off and restart it.
It's almost like engineers know what they're doing.
March 24, 2025
Why are Crazy People Called Mad as a Hatter?
I've know the broad strokes of the mercury-hatter connection for a while but not the specific details and history that are retold in today's video.
Sounds like the hatter/Danbury shakes were a very early version of lack of workplace protections. The hatters might as well have been told to lick their radium paintbrushes.
 |
| Source - vintag.es |
March 17, 2025
Periodic Success: The Hidden Beauty of the Periodic Table
The Royal Institution is a British group founded in 1799 and historically known for promoting and sharing scientific knowledge both within scientific circles and to the general public.
Since 1825 they have been putting on a series of Christmas lectures, many of the most recent of which have been recorded and posted on YouTube for us to see.
This video is the 2014 Christmas lecture going through a fair portion of the periodic table, telling stories about each one and helping us to understand a bit of their arrangement along the way.
March 10, 2025
People said this experiment was impossible, so I tried it
As promised last week, here's the first Veritasium video about thermite.
I'm not thrilled that Dr Derek's titles seem to be getting more clickbaity and less informative. Again this week, the video's title isn't really what the video is about. It's a minor part of the video - here addressed in about six minutes in the middle of the video - and doesn't really cover the bulk of the video's content.
With that being said, seeing thermite in slow motion and through glass is pretty stunning.
Great video...bad title...
March 3, 2025
Why Don’t Railroads Need Expansion Joints?
The title of this video - which might change since I'm writing this up just a day after it was posted to YouTube - is a bit misleading. The actual question in the title - why don't railroads need expansion joints - is only answered in the last half minute or so of the video and is answered more thoroughly in a Practical Engineering video that I'll post after a jump.
The bulk of the video is spent explaining how railroad welds using thermite work. The video explains the nuances far better than other thermite videos I've posted before, explaining why the rails must be aligned and peaked, why the rails must be preheated (including a nice demonstration of heat treating), how the crystal structure changes as a result of the weld, and eventually why the rails don't need expansion joints.
This is the second of at least three thermite videos from Dr Derek. I thought I'd posted the first video to both blogs, but I can't seem to find it, so it'll likely show up next week.
February 24, 2025
The microwave plasma mystery
I've posted about plasma before, specifically from Veritasium. That video does a great job explaining why halved grapes - and other similarly sized, primarily water objects - can create plasma in a microwave oven
Today's video looks at why plasma can be created from non-water systems like matches under an upside-down beaker, something I've tried before and can verify the ease of it working.
Just to protect myself legally, don't try this at home. Be smart, folks.
February 17, 2025
How This 300-Year-Old Pastel Stick Maker Creates Nearly 2,000 Colors — More Than Its Competitors
#forbiddenfrosting
Most hand manufacturing processes are stunning to watch, and in this case it apparently produces a product that is superior to mass manufactured competitors. It has to be way more expensive and time-consuming, though.
So pretty...so mesmerizing...so colorful...
February 10, 2025
The worry about black food plastics...and a correction
This video was published by Adam Ragusea in November 2024 about a study from a month or so earlier than that.
The tl;dr of the study is that many black plastics are produced from recycled black plastics that are frequently sourced from electronic waste which contains higher amounts of particularly toxic, flame-retardant chemicals. Those 'new' black plastic items could - especially if used in high heat areas like food flippers and turners on the stovetop - release higher than safe amounts of those chemicals.
In the above video, Adam goes through the possible concerns that this raises as well as noting a possible math error in the study's calculations suggesting that the level of concern is slightly lower than the authors might have initially suggested.
The article was corrected - noting exactly the math error that Adam suggested, and Adam published a spectacular video explaining why that error should not undermine faith in the scientific process or even in the researchers and authors of the original article.
February 3, 2025
You're Probably Wrong About Rainbows
No, I knew most of that information before. I wasn't wrong.
The new stuff for me is the brilliance of the demonstration showing how the different colors of light have maxima at different angles.
All in all, another great - if increasingly lengthy - video from Dr Derek.
January 31, 2025
Titration humor
Who hasn't been there?
I remember this happening to me in a chemistry lab at Wabash. We didn't got 45 minutes, of course, but I know that we kept thinking there should be a color change by 'now' based on the other titrations we'd done and adding a drop of phenolphthalein just to make sure.
The flask turned bright fuchsia, and we dumped out that trial.
January 27, 2025
LED Christmas lights which don't hurt the eyes: it finally happened!
I'll admit that Alec, the host of Technology Connections, might be a little more bothered than is reasonable about the harshness of blue and green LEDs during the holiday season, but he's at least trying to do something about it. (check the videos after the jump)
In this video, he celebrates the fact that a company is now producing warm white LEDs inside multicolored plastic (or maybe glass) bulb covers resulting in something more akin to the same colors as produced by the incandescent filaments inside colored glass bulbs of yore.
I do agree that the blue and green LEDs can be harsh, and the flicker can bother me at times - don't get me started on the LED bar light in my guest bathroom - but mostly I just grumble and move on.
January 20, 2025
Octet Rule Exceptions Tier List
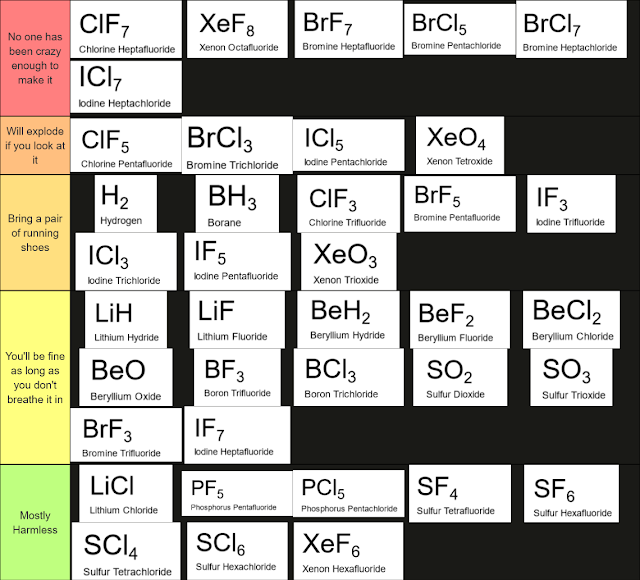 |
| Source - reddit |
The octet rule is a simple 'rule' that generally describes the behavior of elements in compounds: atoms are most stable when they have eight valence electrons.
But like every rule that humanity has come up with to describe the world around us, nature doesn't give even a little thought to following that rule. Nature does whatever nature does and our rules be damned.
As such, the octet rule is followed far more frequently that it is violated, but there certainly are compounds that don't follow the octet rule.
January 13, 2025
Making pop rocks from scratch (is complicated)
Now, as long as he doesn't make cherry coke from from scratch, too, we should be okay.
I love the idea of quasi-carbonating the sugar solution to trap unstable bubbles as it solidifies.
I also love that the large rocks of pop rocks look to be a little dangerous, actually.
I would absolutely eat that big rock.
January 6, 2025
I bought a freeze dryer so you don't have to
This guy does a lot of things that I don't think I should do.
My wife has, admittedly, suggested that she might be interested in buying a freeze dryer. It's not something that's come up more than once or twice, but it's been mentioned.
Clearly buying a freeze dryer would be bonkers and nuts. We don't need it. The energy spent isn't remotely worth the very, very few times we'd ever use it before letting it sit unused in a closet somewhere.
At least that's my perspective on the thing, and clearly Mr. Technology Connections agrees with me.
Even though the science involved is kind of fascinating.









